There's a #NEWTOX in town: Jeuveau™
NEW!! To URBAN YOU : #NEWTOX Jeuveau™
Have you heard?
There’s a #newtox in town
and we’ve got all the info you need!
A new injectable treatment for frown lines is here
Say HELLO to Jeuveau™ ( pronounced Jū vō ) it comes from the french word “nouveau,” which means “new” or “modern” and that’s exactly what it’s meant to do, help you feel new and modern!
We will all get wrinkles at some point in our lives, some of us more and some of us less. These lines can be called “frown lines” and there is a new injectable treatment called Jeuveau™ that works to temporarily improve the appearance of these lines in adults.
Jeuveau™ is a NEW FDA-approved injectable treatment. Jeuveau is made exclusively for aesthetics and improving the appearance of fine lines.
Q. How does it work?
A. Jeuveau™ stops the nerves from telling your face muscles to flex- which has been shown to improve the appearance of frown lines.
Q. What’s it like before and after treatment?
A. If it’s your first time getting a treatment, you may be a little nervous. And that’s okay! Localized pain, infection, inflammation, tenderness, swelling, erythema, and/or bleeding/bruising may be associated with the injection.
For specific instructions on what to do before and after your injection, please follow the instructions as given to you by your healthcare provider.
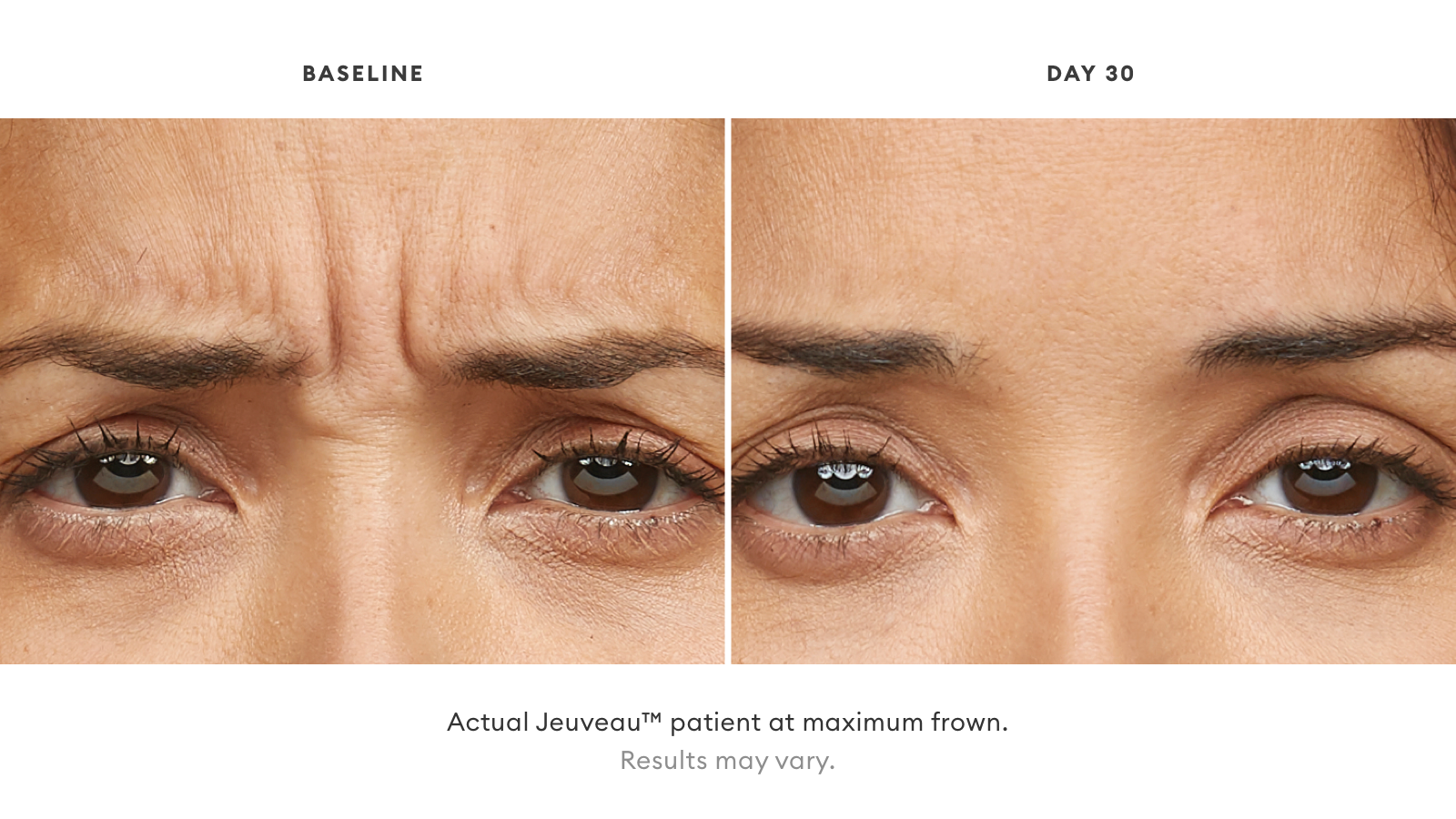
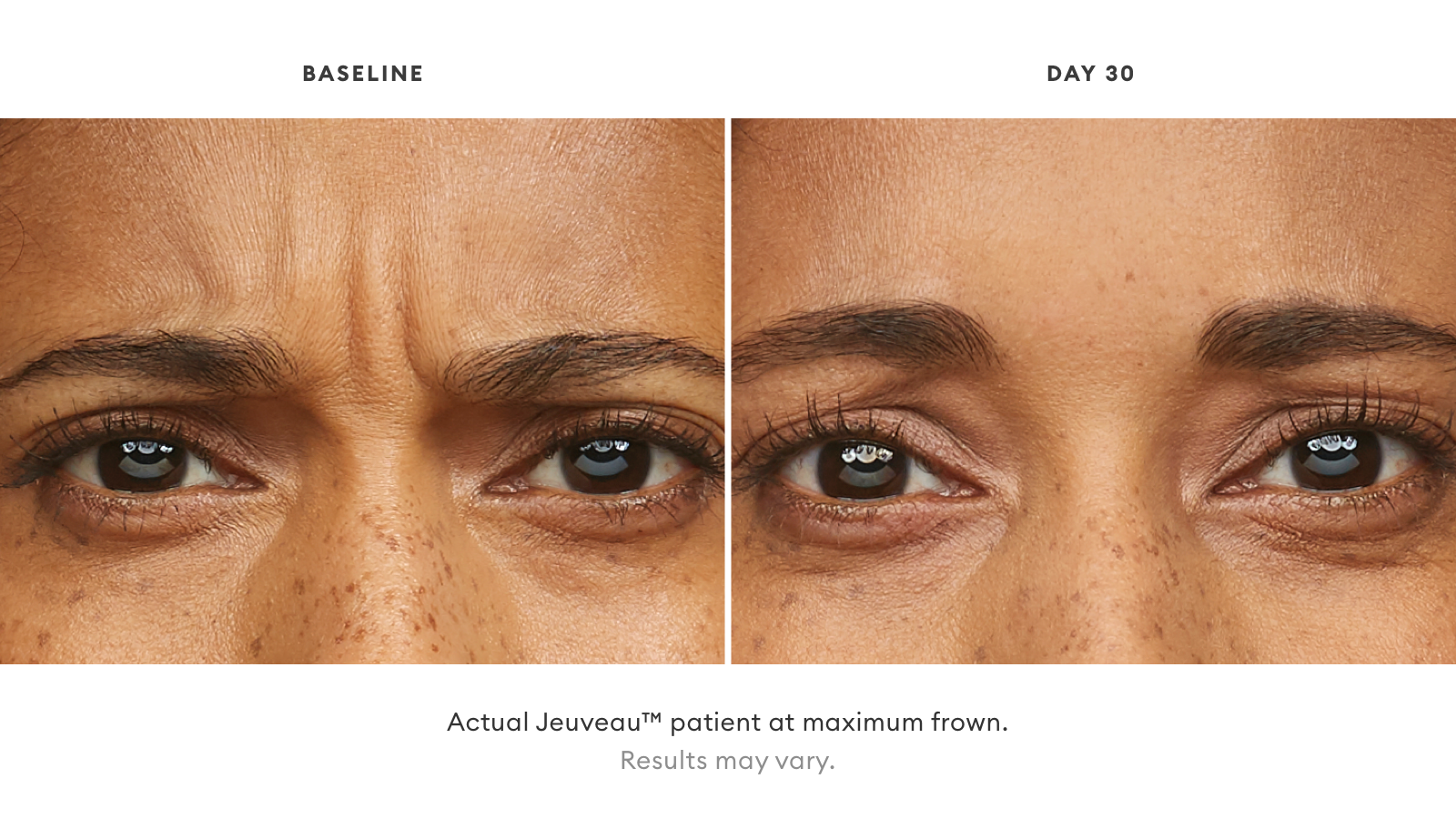
Check out Before and Afters from Jeuveau™ below:
Enjoy a $75 savings on 30 or more units of Jeuveau treatment with #NEWTOX Now - don’t miss your chance to take advantage of this exclusive promotion!
IMPORTANT SAFETY INFORMATION FOR JEUVEAU™ (prabotulinumtoxinA-xvfs)
JEUVEAU may cause serious side effects that can be life threatening. Get medical help right away if you have any of these problems any time (hours to weeks) after injection of JEUVEAU:
Problems swallowing, speaking, or breathing, due to weakening of associated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including: loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing.
Do not use JEUVEAU if you: are allergic to any of the ingredients in JEUVEAU (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®/BOTOX® Cosmetic), abobotulinumtoxinA (DYSPORT®), or incobotulinumtoxinA (XEOMIN®); have a skin infection at the planned injection site; or are a child.
JEUVEAU dosing units are not the same as, or comparable to, any other botulinum.
Tell your healthcare provider about all your muscle or nerve conditions, such as ALS or Lou Gehrig’s disease, Myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects including difficulty swallowing and difficulty breathing from typical doses of JEUVEAU.
Tell your healthcare provider about all your medical conditions, including: any side effects from botulinum toxin products, including dry eye; breathing, swallowing, bleeding, or heart problems; plans to have surgery; weakness of forehead muscles; drooping eyelids; had surgery on your face; are pregnant or breastfeeding or plan to become pregnant or breastfeed (it is not known if JEUVEAU can harm your unborn baby or passes into breast milk).
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using JEUVEAU with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your healthcare provider that you have received JEUVEAU in the past.
Especially tell your healthcare provider if you: have received any other botulinum toxin product in the past and the last 4 months. and exactly which product you received (such as BOTOX, BOTOX Cosmetic, MYOBLOC, DYSPORT, or XEOMIN).
JEUVEAU may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of treatment with JEUVEAU. If this happens, do not drive a car, operate machinery, or do other dangerous activities.
JEUVEAU can cause other serious side effects including: allergic reactions (such as itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint), heart problems (such as irregular heartbeat and heart attack), and eye problems (including dry eye, reduced blinking, and corneal problems). Tell your healthcare provider or get medical emergency help right away if you experience a serious side effect.
The most common side effects include: headache; eyelid drooping, upper respiratory tract infection, and increased white blood cell count in your blood.
APPROVED USE
JEUVEAU is a prescription medicine that is injected into muscles and used in adults for a short period of time (temporary) to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines).
The risk information provided here is not complete. For more information about JEUVEAU, see the full Prescribing Informationincluding BOXED WARNING, and Medication Guide, or talk to your healthcare provider.
To report side effects associated with use of JEUVEAU, please call 877-EVOLUS1 (877-386-5871). You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
REFERENCES: 1. Jeuveau [Package Insert], Santa Barbara, CA: Evolus, Inc; 2019. 2. United States Patent: US 9,512,418 B2; Dec. 6, 2016. 3. Data on File, CSR EV-001, BLA761085, Evolus, Inc., Santa Barbara, CA. 4. Data on File, CSR EV-002, BLA761085, Evolus, Inc., Santa Barbara, CA. 5. Data on File, CSR EVB-003, BLA761085, Evolus, Inc., Santa Barbara, CA. 6. Data on File, CSR EV-004, BLA761085, Evolus, Inc., Santa Barbara, CA. 7. Data on File, CSR EV-006, BLA761085, Evolus, Inc., Santa Barbara, CA.
Manufactured by: Evolus, Inc., 1027 Garden St., Santa Barbara, CA 93101
©2019 Evolus, Inc. All rights reserved. JEUVEAU is a trademark of Evolus, Inc. All other trademarks are the property of their respective owners.