Hello, Jeuveau!
JeuveauTM (prabotulinumtoxinA-xvfs) by Evolus is now FDA approved for the temporary improvement of moderate to severe glabellar lines in adults.
Jeuveau™ is the first aesthetic-only neurotoxin approved in the U.S.
About Jeuveau™
Jeuveau™ (prabotulinumtoxinA-xvfs) is a proprietary 900 kDa purified botulinum toxin type A formulation indicated for the temporary improvement in the appearance of moderate to severe glabellar lines in adults. Jeuveau™ is produced under strict quality and safety standards in a state-of-the art facility, specifically built to manufacture Jeuveau™. The safety and efficacy of Jeuveau™ has been evaluated in clinical studies with over 2,100 patients enrolled.
FDA approval of Jeuveau™ was supported by clinical data from two U.S. Phase III randomized, multi-center, double-blind, placebo-controlled clinical trials both of which met the primary endpoint and demonstrated efficacy compared with placebo in the reduction of the severity of glabellar lines, defined as a 2-point composite improvement agreed upon by physician and patient, at Day 30. 67.5% of subjects in study one (EV-001) and 70.4% of subjects in study two (EV-002) met the primary endpoint, compared to 1.2% and 1.3% of patients in each placebo arm respectively.
#NEWTOX BEFORE & AFTER
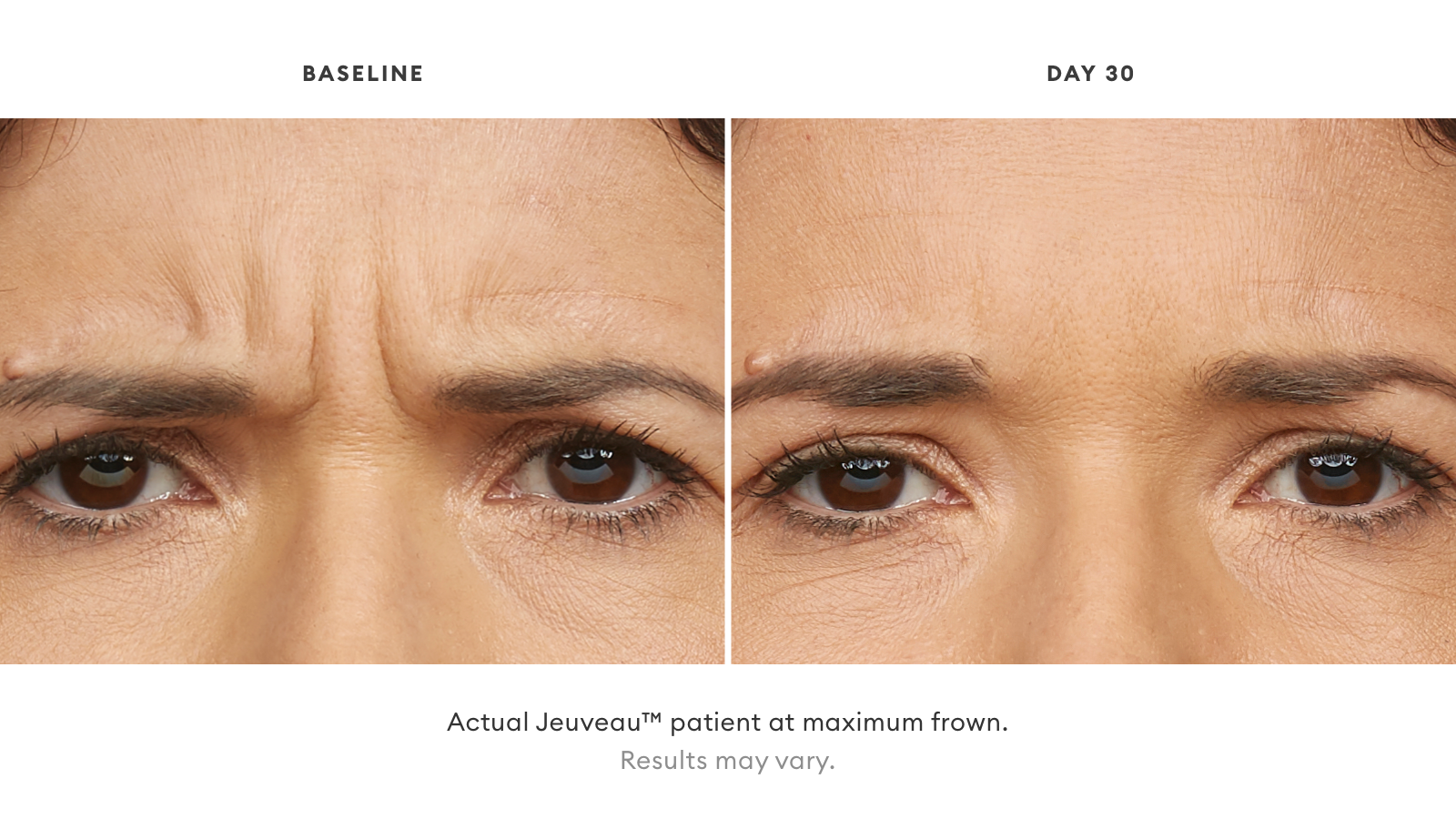


Want to find out if Jeuveau is right for you?
Call 616-570-0228 to set up a complimentary consultation with on of our experienced injectionists.

