Learn more about our medical spa services at Urban You Beauty Bar
JUVÉDERM Ultra & Ultra Plus®xc in Grand Rapids
at Urban You Beauty Bar
JUVÉDERM® XC is proven to last up to 1 year in moderate to severe facial wrinkles and lines.*
JUVÉDERM® Ultra XC is the first filler proven to last up to 1 year in the lips.*
Over time, your skin loses elasticity. This natural process, plus genetics and environmental factors like sun exposure, can cause moderate to severe lines and folds such as parentheses, corner, and marionette lines to form around the nose and mouth. JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC can help fill these lines and give you natural-looking results that last.
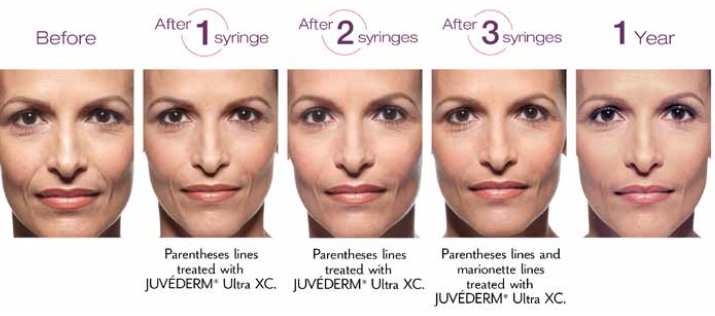
JUVÉDERM ultra & ultra plus®XC: Before & After
Actual patient. Results may vary. Unretouched photos taken before treatment, after a 3-syringe treatment, and 1 year after treatment with JUVÉDERM® XC. One syringe = 1.0 mL of JUVÉDERM® XC. A total of 3.0 mL of JUVÉDERM® XC was injected into the parentheses and marionette lines.
Actual patient. Results may vary. Unretouched photos taken before treatment and 2 weeks after treatment with JUVÉDERM® XC. A total of 3.2 mL of JUVÉDERM® XC was injected into the parentheses, marionette, and corner lines.
Actual patient. Results may vary. Unretouched photos taken before treatment and 1 month after treatment with 1.5 mL of JUVÉDERM® Ultra XC in the lips.
Actual patient. Results may vary. Unretouched photos taken before treatment and 1 month after treatment with 1.2 mL of JUVÉDERM® Ultra XC in the lips.
Click to View Important Safety Information
JUVÉDERM® Ultra XC Indication and Important Safety Information
Indication
JUVÉDERM® Ultra XC injectable gel is indicated for injection into the lips and perioral area for lip augmentation in adults over the age of 21.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
This product should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to gram-positive bacterial proteins or lidocaine contained in this product.
WARNINGS
• Do not inject into blood vessels. Introduction of JUVÉDERM® Ultra XC into the vasculature may lead to embolization, occlusion of the vessels, ischemia,
or infarction. Take extra care when injecting soft-tissue fillers; for example, inject the product slowly and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary
or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms: changes in vision, signs of a stroke, blanching of the skin, unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and, possibly, evaluation by an appropriate healthcare professional specialist should an intravascular injection occur
• Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled
PRECAUTIONS
• In order to minimize the risk of potential complications, this product should only be used by healthcare professionals who have appropriate training, experience, and who are knowledgeable about facial anatomy
• Healthcare professionals are encouraged to discuss the potential risks of soft-tissue injections with their patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications
• The safety and effectiveness for the treatment of anatomic regions other than moderate to severe facial wrinkles and folds, and lips and perioral area for lip augmentation, have not been established in controlled clinical studies
• As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Follow standard precautions associated with injectable materials
• The safety for use during pregnancy, in breastfeeding females, and in patients under 18 years has not been established
• The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied
• Use with caution in patients on immunosuppressive therapy
• Patients who are using products that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may experience increased bruising or bleeding at treatment sites
ADVERSE EVENTS
The most commonly reported side effects are temporary injection-site redness, swelling, pain/tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. Most side effects are mild or moderate in nature, lasting 14 days or less.
To report a problem with JUVÉDERM® Ultra XC, please call Allergan Product Surveillance at 1-800-624-4261.
For more information, please see JuvedermDFU.com or call the Allergan Medical Information line at 1-800-433-8871. JUVÉDERM® Ultra XC injectable gel is available by prescription only.
APPROVED USE JUVÉDERM® Ultra XC injectable gel is for injection into and around the lips for lip augmentation in adults over 21.
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive JUVÉDERM® Ultra XC?
Do not use if you have a history of multiple severe allergies or severe allergic reactions (anaphylaxis), or if you are allergic to lidocaine or the gram-positive bacterial proteins used in this product.
What precautions should my doctor advise me about?
• Tell your doctor if you are pregnant or breastfeeding. The safety for use during pregnancy or in women who are breastfeeding has not been studied
• The safety for use in patients under 18 years has not been studied
• The safety and effectiveness of JUVÉDERM® Ultra XC for treatment in areas other than facial
wrinkles and folds, or in the lips and perioral area for lip augmentation, have not been established
in clinical studies
• Tell your doctor if you have a history of excessive scarring (eg, hypertrophic scarring and keloid
formations) or pigmentation disorders, as use of this product may result in additional scars or
changes in pigmentation
• Tell your doctor if you are planning other laser treatments or a chemical peel, as there is a possible
risk of inflammation at the treatment site if these procedures are performed after treatment
• Patients who experience skin injury near the site of injection with this product may be at a higher
risk for side effects
• Tell your doctor if you are on immunosuppressive therapy used to decrease the body’s immune response, as use of this product may result in an increased risk of infection
• Tell your doctor if you are using medications that can prolong bleeding, such as aspirin, ibuprofen, or other blood thinners, as this may result in increased bruising or bleeding at the injection site
• Minimize strenuous exercise, exposure to extensive sun or heat, and alcoholic beverages within the first 24 hours following treatment
What are possible side effects?
The most common side effects with JUVÉDERM® Ultra XC include tenderness, swelling, firmness, lumps/ bumps, bruising, pain, redness, discoloration, and itching. Most side effects are mild or moderate and last 14 days or less.
One of the risks with using this product is unintentional injection into a blood vessel, and while rare, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring.
As with all skin injection procedures, there is a risk of infection.
To report a side effect with JUVÉDERM® Ultra XC, please call Allergan Product Surveillance at
1-800-624-4261.
For more information, please see Juvederm.com or call Allergan Medical Information at 1-800-433-8871.
Available by prescription only.
JUVÉDERM® XC Important Information
INDICATION
JUVÉDERM® XC injectable gels (JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC) are indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
These products should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to gram-positive bacterial proteins or lidocaine contained in these products.
WARNINGS
• Do not inject into blood vessels. Introduction of these products into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft-tissue fillers; for example, inject the product slowly and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary
or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms: changes in vision, signs of a stroke, blanching of the skin, unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and, possibly, evaluation by an appropriate healthcare professional specialist should an intravascular injection occur
• Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled
PRECAUTIONS
• In order to minimize the risk of potential complications, JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC injectable gels should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy
• Healthcare professionals are encouraged to discuss the potential risks of soft-tissue injections with their patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications
• The safety and effectiveness for the treatment of anatomic regions other than moderate to severe facial wrinkles and folds with JUVÉDERM® XC, and the lips and perioral area for lip augmentation with JUVÉDERM® Ultra XC, have not been established in controlled clinical studies
• As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Follow standard precautions associated with injectable materials
• The safety for use during pregnancy, in breastfeeding females, and in patients under 18 years has not been established
• The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied
• Use with caution in patients on immunosuppressive therapy
• Patients who are using products that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may experience increased bruising or bleeding at treatment sites
ADVERSE EVENTS
The most commonly reported side effects are temporary injection-site redness, swelling, pain/tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. Most side effects are mild or moderate in nature, lasting 14 days or less.
To report a problem with JUVÉDERM® Ultra XC or JUVÉDERM® Ultra Plus XC, please call Allergan Product Surveillance at 1-800-624-4261.
For more information, please see JuvedermDFU.com or call the Allergan Medical Information line at 1-800-433-8871. JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC injectable gels are available by prescription only.
People who were interested in JUVÉDERM ULTRA might also be interested in: BOTOX® COSMETIC, JUVÉDERM VOLUMA®XC, JUVÉDERM VOLBELLA®, JUVÉDERM VOLLURE™ & KYBELLA